PK Profiles of IFX IV and IFX SC
Theoretical PK profiles of IFX (CT-P13) IV vs SC1
Chart adapted from Little RD, et al. J Clin Med. 2022;11(20):6173.1
Study Note: This chart reflects hypothetical serum drug levels for illustrative purposes. The chart was derived from a comprehensive narrative review by Little et al., which synthesized findings from multiple studies. The review predominantly focused on studies involving CT-P13, administered both IV and SC.1
Current evidence does not establish a direct correlation between the pharmacokinetic attributes and clinical efficacy. The prognostic relevance of serum drug concentrations should be interpreted within the complete clinical context, not as the sole predictor of therapeutic outcomes.1
Mean IFX serum concentration: SC vs IV in patients with IBD2
Study Note: This graph illustrates the pharmacokinetic profiles from Study 1.6, an open-label, multicenter, parallel-subset Phase 1 randomized controlled trial (RCT) that compared SC and IV administration of IFX in a population of patients with IBD. The analysis compared serum infliximab concentrations of SC 120 mg Q2W and IV 5 mg/kg Q8W, including evaluation after patients in the IV arm shifted to SC at Week 30. The objective was to provide data focusing on the subset of patients with IBD who received the FDA-approved dosing of IFX SC 120 mg Q2W in part 2 of the 1.6 study.2
Current evidence does not establish a direct correlation between the pharmacokinetic attributes and clinical efficacy. The diagnostic relevance of serum drug concentrations should be interpreted within the complete clinical context, not as the sole predictor of therapeutic outcomes.2
*Patients in the IFX-dyyb IV 5 mg/kg Q8W subset shifted to IFX-dyyb SC 120 mg Q2W at Week 30.
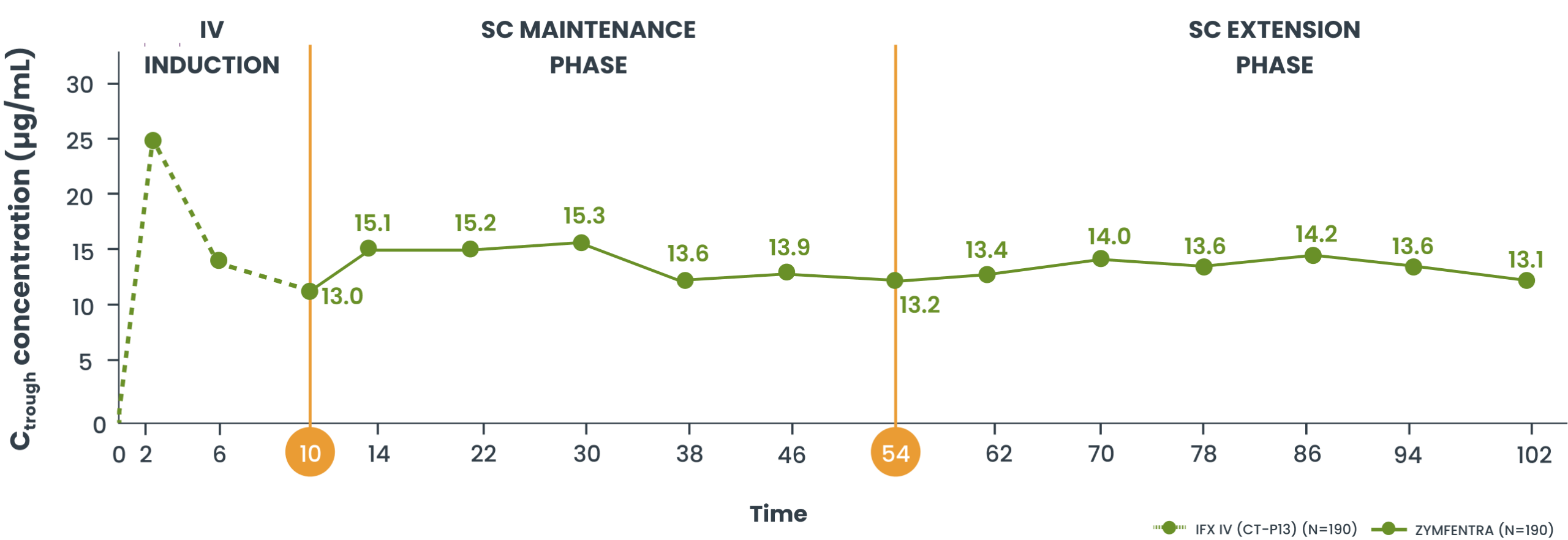
Serum drug levels through Year 2 in patients with CD3
- Post-Week 10, serum concentrations in the ZYMFENTRA group rose until Week 14 and stayed stable through Week 54 due to biweekly dosing
- During the SC Maintenance and Extension phases, mean range (CV%) of observed trough was 13.1 µg/mL to 15.3 µg/mL in the ZYMFENTRA group
Mean serum concentration in patients with CD3
Study Note: From Week 0 through Week 10, patients were in the induction phase with IFX IV. In the treatment group, post-Week 10, patients were given ZYMFENTRA for maintenance. During Week 22 through Week 54, dose adjustments were allowed for patients who initially responded but then lost response according to the loss of response criteria.3
Current evidence does not establish a direct correlation between the pharmacokinetic attributes of ZYMFENTRA and its clinical efficacy. The prognostic relevance of serum drug concentrations should be interpreted within the complete clinical context, not as the sole predictor of therapeutic outcomes.3
CD, Crohn's disease; CV%, coefficient of variation (standard deviation as a percentage of the mean); IBS, irritable bowel syndrome; IFX, infliximab; IV, intravenous; PK, pharmacokinetic; Q2W, every 2 weeks; Q8W, every 8 weeks; SC, subcutaneous; UC, ulcerative colitis.
References: 1. Little RD, Ward MG, Wright E, et al. Therapeutic drug monitoring of subcutaneous infliximab in inflammatory bowel disease—understanding pharmacokinetics and exposure response relationships in a new era of subcutaneous biologics. J Clin Med. 2022;11(6173). doi: 10.3390/jcm11206173 2. Data on file. Celltrion USA, Inc. 3. Colombel J-F, Sandborn WJ, Schreiber S. Supplementary material to: Subcutaneous infliximab (CT-P13 SC) as maintenance therapy for Crohn's disease and ulcerative colitis: 2-year results from open-label extensions of two randomized controlled trials (LIBERTY). J Crohns Colitis. 2025;19(6):1-15. doi: 10.1093/ecco-jcc/jjaf060
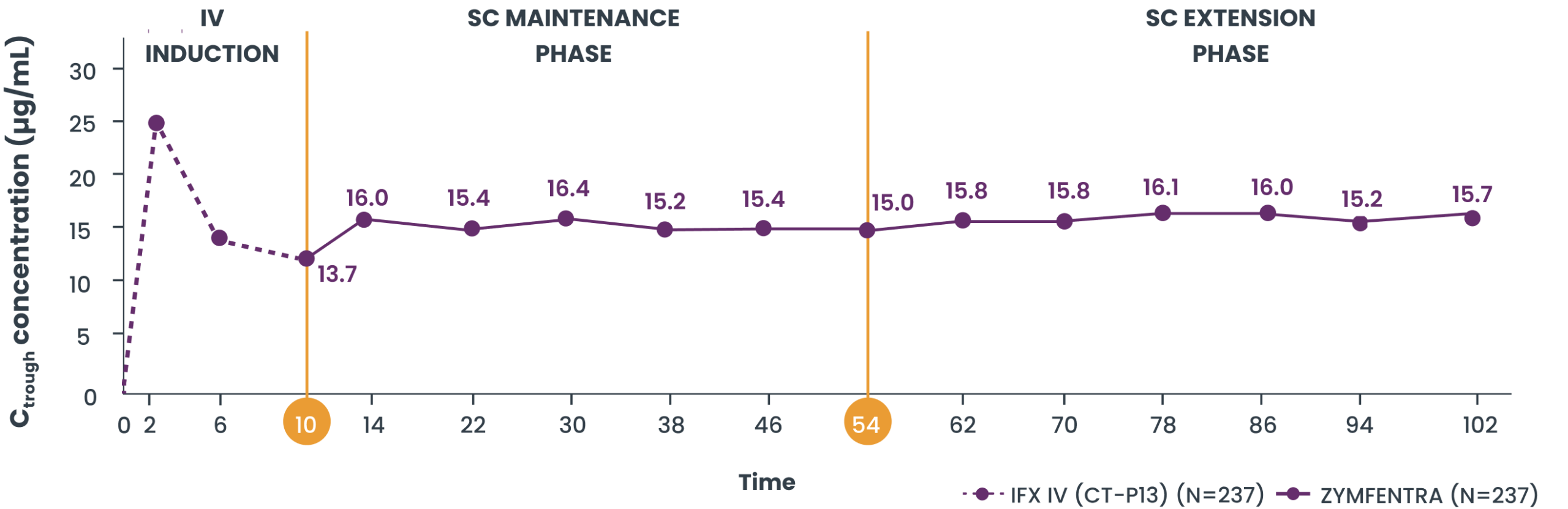
Serum drug levels through Year 2 in patients with UC3
- Post-Week 10, serum concentrations in the ZYMFENTRA group stayed stable through Week 102 due to biweekly dosing
- During the SC Maintenance and Extension phases, mean range (CV%) of observed trough was 15.0 µg/mL to 16.4 µg/mL in the ZYMFENTRA group
Mean serum concentration in patients with UC3
Study Note: From Week 0 through Week 10, patients were in the induction phase with IFX IV. In the treatment group, post-Week 10, patients were given ZYMFENTRA for maintenance. During Week 22 through Week 54, dose adjustments were allowed for patients who initially responded but then lost response according to the loss of response criteria.3
Current evidence does not establish a direct correlation between the pharmacokinetic attributes of ZYMFENTRA and its clinical efficacy. The prognostic relevance of serum drug concentrations should be interpreted within the complete clinical context, not as the sole predictor of therapeutic outcomes.3
CD, Crohn's disease; CV%, coefficient of variation (standard deviation as a percentage of the mean); IBS, irritable bowel syndrome; IFX, infliximab; IV, intravenous; PK, pharmacokinetic; Q2W, every 2 weeks; Q8W, every 8 weeks; SC, subcutaneous; UC, ulcerative colitis.
References: 1. Little RD, Ward MG, Wright E, et al. Therapeutic drug monitoring of subcutaneous infliximab in inflammatory bowel disease—understanding pharmacokinetics and exposure response relationships in a new era of subcutaneous biologics. J Clin Med. 2022;11(6173). doi: 10.3390/jcm11206173 2. Data on file. Celltrion USA, Inc. 3. Colombel J-F, Sandborn WJ, Schreiber S. Supplementary material to: Subcutaneous infliximab (CT-P13 SC) as maintenance therapy for Crohn's disease and ulcerative colitis: 2-year results from open-label extensions of two randomized controlled trials (LIBERTY). J Crohns Colitis. 2025;19(6):1-15. doi: 10.1093/ecco-jcc/jjaf060
IMPORTANT SAFETY INFORMATION AND INDICATIONS
WARNING: SERIOUS INFECTIONS and MALIGNANCY
SERIOUS INFECTIONS
Patients treated with TNF blockers, including ZYMFENTRA, are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
Discontinue ZYMFENTRA if a patient develops a serious infection or sepsis.
Reported infections include:
- Active tuberculosis, including reactivation of latent tuberculosis. Patients with tuberculosis have frequently presented with disseminated or extrapulmonary disease. Test patients for latent tuberculosis before ZYMFENTRA use and during therapy. Initiate treatment for latent infection prior to ZYMFENTRA use.
- Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Consider empiric anti-fungal therapy in patients at risk for invasive fungal infections who develop severe systemic illness.
- Bacterial, viral and other infections due to opportunistic pathogens, including Legionella and Listeria.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with ZYMFENTRA, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
MALIGNANCY
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including infliximab products.
Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma, have been reported in patients treated with TNF blockers, including infliximab products. These cases have had a very aggressive disease course and have been fatal. Almost all patients had received treatment with azathioprine or 6-mercaptopurine concomitantly with a TNF blocker at or prior to diagnosis. The majority of reported cases have occurred in patients with Crohn’s disease or ulcerative colitis and most were in young adult males.
Contraindications
- ZYMFENTRA is contraindicated in patients with a history of a severe hypersensitivity reaction to infliximab-dyyb, other infliximab products, any of the inactive ingredients in ZYMFENTRA, or any murine proteins. Reactions have included anaphylaxis.
Warnings and Precautions
- Serious infections: Avoid in patients with active infection. If infection develops, conduct a prompt/complete diagnostic workup appropriate for immunocompromised patients and initiate antimicrobials. If systemic illness develops in patients who reside or travel to regions where mycoses are endemic, consider empiric antifungals.
- Malignancies: Malignancies, including lymphoma, were greater in TNF-blocker-treated patients. Consider the higher risk of hepatosplenic T-cell lymphoma (HSTCL) with combination therapy versus increased risk of immunogenicity and hypersensitivity reactions with monotherapy.
- Hepatitis B virus (HBV) reactivation: Test for HBV infection before starting treatment. Monitor HBV carriers during and several months after therapy for active HBV infection. If reactivation occurs, stop ZYMFENTRA and begin anti-viral therapy.
- Hepatotoxicity: Severe hepatic reactions, some fatal or necessitating liver transplantation have occurred in patients receiving infliximab products. Monitor hepatic enzymes and liver function tests every 3-4 months during treatment; investigate liver enzyme elevations and interrupt treatment if drug-induced liver injury is suspected. Instruct patients to seek immediate medical attention if symptoms develop.
- Congestive heart failure (CHF): New onset or worsening symptoms may occur. Avoid in patients with CHF. Monitor for new/worsening symptoms when administering ZYMFENTRA.
- Hematologic reactions: Advise patients to seek immediate medical attention if signs and symptoms of cytopenia develop; consider stopping if significant hematologic abnormalities develop.
- Hypersensitivity and other administration reactions: Serious hypersensitivity reactions, including anaphylaxis have occurred with intravenous formulations of infliximab; discontinue ZYMFENTRA and start appropriate therapy.
- Neurologic reactions: Exacerbation or new onset CNS demyelinating disorders may occur; consider discontinuation of ZYMFENTRA.
- Risk of infection with concurrent administration of other biological products: Concurrent use with other immunosuppressive biologics may increase risk of infection.
- Risk of additive immunosuppressive effects from prior biological products: Consider the half-life and mode of action of prior biologics.
- Autoimmunity: Formation of autoantibodies and development of lupus-like syndrome may occur; discontinue ZYMFENTRA if symptoms develop.
- Vaccinations and use of live vaccines/therapeutic infectious agents: Prior to initiating ZYMFENTRA bring patients up to date with vaccinations. Live vaccines or therapeutic infectious agents should not be given with ZYMFENTRA. A 6-month waiting period following birth is recommended before the administration of live vaccines to infants exposed in utero to infliximab.
Common Adverse Reactions (≥3%)
- Ulcerative Colitis: COVID-19, anemia, arthralgia, injection site reaction, increased alanine aminotransferase, and abdominal pain.
- Crohn's Disease: COVID-19, headache, upper respiratory tract infection, injection site reaction, diarrhea, increased blood creatine phosphokinase, arthralgia, increased alanine aminotransferase, hypertension, urinary tract infection, neutropenia, dizziness, and leukopenia.
Drug Interactions
- Concurrent use with immunosuppressive biologics used to treat UC and CD is not recommended due to risk of infection.
- Formation of CYP450 enzymes may be suppressed by increased levels of cytokines during chronic inflammation. ZYMFENTRA could normalize the formation of CYP450 enzymes potentially resulting in decreased exposure of CYP450 substrates and requiring dose adjustments.
INDICATIONS
Crohn's Disease
- ZYMFENTRA is indicated in adults for maintenance treatment of moderately to severely active Crohn's disease following treatment with an infliximab product administered intravenously.
Ulcerative Colitis
- ZYMFENTRA is indicated in adults for maintenance treatment of moderately to severely active ulcerative colitis following treatment with an infliximab product administered intravenously.
Please see full Prescribing Information, including BOXED WARNING.
INDICATIONS
Crohn's Disease
- ZYMFENTRA is indicated in adults for maintenance treatment of moderately to severely active Crohn's disease following treatment with an infliximab product administered intravenously.
Ulcerative Colitis
- ZYMFENTRA is indicated in adults for maintenance treatment of moderately to severely active ulcerative colitis following treatment with an infliximab product administered intravenously.
IMPORTANT SAFETY INFORMATION AND INDICATIONS
WARNING: SERIOUS INFECTIONS and MALIGNANCY
SERIOUS INFECTIONS
Patients treated with TNF blockers, including ZYMFENTRA, are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
Discontinue ZYMFENTRA if a patient develops a serious infection or sepsis.
Reported infections include:
- Active tuberculosis, including reactivation of latent tuberculosis. Patients with tuberculosis have frequently presented with disseminated or extrapulmonary disease. Test patients for latent tuberculosis before ZYMFENTRA use and during therapy. Initiate treatment for latent infection prior to ZYMFENTRA use.
- Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Consider empiric anti-fungal therapy in patients at risk for invasive fungal infections who develop severe systemic illness.
- Bacterial, viral and other infections due to opportunistic pathogens, including Legionella and Listeria.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with ZYMFENTRA, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
MALIGNANCY
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including infliximab products.
Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma, have been reported in patients treated with TNF blockers, including infliximab products. These cases have had a very aggressive disease course and have been fatal. Almost all patients had received treatment with azathioprine or 6-mercaptopurine concomitantly with a TNF blocker at or prior to diagnosis. The majority of reported cases have occurred in patients with Crohn’s disease or ulcerative colitis and most were in young adult males.
Contraindications
- ZYMFENTRA is contraindicated in patients with a history of a severe hypersensitivity reaction to infliximab-dyyb, other infliximab products, any of the inactive ingredients in ZYMFENTRA, or any murine proteins. Reactions have included anaphylaxis.
Warnings and Precautions
- Serious infections: Avoid in patients with active infection. If infection develops, conduct a prompt/complete diagnostic workup appropriate for immunocompromised patients and initiate antimicrobials. If systemic illness develops in patients who reside or travel to regions where mycoses are endemic, consider empiric antifungals.
- Malignancies: Malignancies, including lymphoma, were greater in TNF-blocker-treated patients. Consider the higher risk of hepatosplenic T-cell lymphoma (HSTCL) with combination therapy versus increased risk of immunogenicity and hypersensitivity reactions with monotherapy.
- Hepatitis B virus (HBV) reactivation: Test for HBV infection before starting treatment. Monitor HBV carriers during and several months after therapy for active HBV infection. If reactivation occurs, stop ZYMFENTRA and begin anti-viral therapy.
- Hepatotoxicity: Severe hepatic reactions, some fatal or necessitating liver transplantation have occurred in patients receiving infliximab products. Monitor hepatic enzymes and liver function tests every 3-4 months during treatment; investigate liver enzyme elevations and interrupt treatment if drug-induced liver injury is suspected. Instruct patients to seek immediate medical attention if symptoms develop.
- Congestive heart failure (CHF): New onset or worsening symptoms may occur. Avoid in patients with CHF. Monitor for new/worsening symptoms when administering ZYMFENTRA.
- Hematologic reactions: Advise patients to seek immediate medical attention if signs and symptoms of cytopenia develop; consider stopping if significant hematologic abnormalities develop.
- Hypersensitivity and other administration reactions: Serious hypersensitivity reactions, including anaphylaxis have occurred with intravenous formulations of infliximab; discontinue ZYMFENTRA and start appropriate therapy.
- Neurologic reactions: Exacerbation or new onset CNS demyelinating disorders may occur; consider discontinuation of ZYMFENTRA.
- Risk of infection with concurrent administration of other biological products: Concurrent use with other immunosuppressive biologics may increase risk of infection.
- Risk of additive immunosuppressive effects from prior biological products: Consider the half-life and mode of action of prior biologics.
- Autoimmunity: Formation of autoantibodies and development of lupus-like syndrome may occur; discontinue ZYMFENTRA if symptoms develop.
- Vaccinations and use of live vaccines/therapeutic infectious agents: Prior to initiating ZYMFENTRA bring patients up to date with vaccinations. Live vaccines or therapeutic infectious agents should not be given with ZYMFENTRA. A 6-month waiting period following birth is recommended before the administration of live vaccines to infants exposed in utero to infliximab.
Common Adverse Reactions (≥3%)
- Ulcerative Colitis: COVID-19, anemia, arthralgia, injection site reaction, increased alanine aminotransferase, and abdominal pain.
- Crohn's Disease: COVID-19, headache, upper respiratory tract infection, injection site reaction, diarrhea, increased blood creatine phosphokinase, arthralgia, increased alanine aminotransferase, hypertension, urinary tract infection, neutropenia, dizziness, and leukopenia.
Drug Interactions
- Concurrent use with immunosuppressive biologics used to treat UC and CD is not recommended due to risk of infection.
- Formation of CYP450 enzymes may be suppressed by increased levels of cytokines during chronic inflammation. ZYMFENTRA could normalize the formation of CYP450 enzymes potentially resulting in decreased exposure of CYP450 substrates and requiring dose adjustments.
INDICATIONS
Crohn's Disease
- ZYMFENTRA is indicated in adults for maintenance treatment of moderately to severely active Crohn's disease following treatment with an infliximab product administered intravenously.
Ulcerative Colitis
- ZYMFENTRA is indicated in adults for maintenance treatment of moderately to severely active ulcerative colitis following treatment with an infliximab product administered intravenously.
Please see full Prescribing Information, including BOXED WARNING.